
A Not-So-Straightforward Case of Endometriosis
December 7, 2020
From the December 2020 Issue of Clinical Advisor
A Not-So-Straightforward Case of Endometriosis
A 26-year-old White woman presents to the gynecology clinic complaining of a 6-month history of progressively worsening right lower quadrant (RLQ) pain and painful defecation. She reports constant, dull, and throbbing pain, with occasional sharp, stabbing pains that radiate to her lower back and right hip. She also describes a “pulling sensation” in her abdomen during defecation.
Her symptoms include fatigue, constipation, bloating, decreased stool caliber, pressure on urination, and deep dyspareunia. The patient notes that symptoms are notably worse the week before menses. She denies weight loss, hematochezia, fever, nausea, vomiting, hematuria, vaginal discharge, dysmenorrhea, and menorrhagia and reports that ibuprofen and polyethylene glycol laxatives have not relieved her pain.
Medical History
Medical history is significant for a hemorrhagic right ovarian cyst that resulted in an emergency department visit 4 months before the onset of the current symptoms. She is an otherwise healthy woman, with no history of pregnancy, abdominal surgery, or sexually transmitted disease. The patient’s menarche occurred at 13 years of age, and her menstrual cycles occur every 28 to 32 days and last 5 to 7 days. Her family history is positive for uterine fibroids and breast and colon cancers. She takes no medications other than those previously mentioned for pain and constipation.
On further questioning, the patient admits the dyspareunia began when she was 22 years old. She reports that 3 different gynecologists examined her and failed to identify a cause for her dyspareunia. She was referred to physical therapy for pelvic floor strengthening but was dismissed after her first treatment due to normal pelvic tone. No other treatments or diagnostic tests were offered.
Around this time, she also had 2 visits to her primary care provider (PCP) for evaluation of her abdominal pain and gastrointestinal complaints. Her examinations were unremarkable and a hemoccult test was negative. The PCP recommended an over-the-counter laxative and told her to eat a higher fiber diet for her constipation. The PCP also advised her to try a gluten and lactose elimination diet to rule out food allergies. The patient’s symptoms persisted despite these therapies. Her PCP ultimately recommended that she follow up with a gynecology provider due to her recent history of an ovarian cyst.
Gynecologic Examination and Imaging
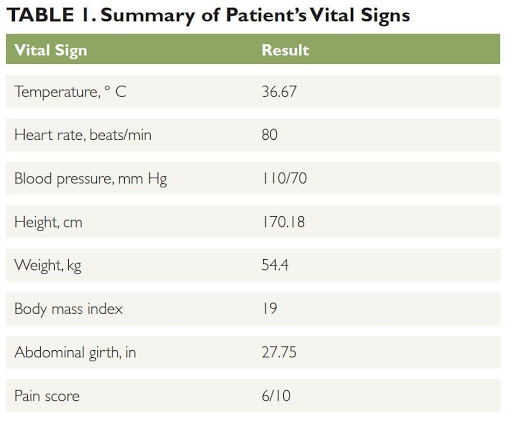
On examination, the patient’s abdomen is soft and nondistended, with bowel sounds active in all 4 quadrants. She has moderate tenderness and guarding during palpation of the right lower quadrant. No abdominal mass is noted and costovertebral angle tenderness is negative. Her vitals are shown in Table 1.
During pelvic examination, the patient experiences mild pain on insertion of the vaginal speculum. Her external genitalia, vaginal mucosa, and cervix appear normal. The adnexa of the uterus are moderately tender on bimanual examination. She does not have cervical motion tenderness. Palpation of the uterosacral ligaments and posterior cul-de-sac during rectovaginal examination causes severe pain, and there is palpable fullness in the posterior cul-de-sac. A hemoccult test is negative.
On transvaginal ultrasound (TVUS), the uterus is anteverted and measures 8.50 × 4.27 × 4.15 cm, with an endometrial thickness of 0.73 cm. The myometrium appears normal. A small amount of fluid is present in the cul-de-sac. The bladder is compressed by the uterus. The left ovary is located laterally and measures 5.43 × 3.71 × 4.07 cm. It contains multiple simple follicles. No abnormal blood flow is noted. The right ovary is enlarged by an 8.08 × 8.19 × 7.03 cm cyst with a wall thickness of 0.55 cm. The cyst appears homogeneous and hypoechoic, surrounded by poor vascular flow.
Assessment and Plan
Differential diagnosis of the patient’s pelvic pain and right adnexal mass includes endometriosis, ovarian cyst, and ovarian tumor. She was offered diagnostic laparoscopy for further assessment. Multiple preoperative laboratory studies were ordered, including urinalysis and culture (UA&C), urine human chorionic gonadotropin (HCG), complete blood count with differential, complete metabolic panel, testosterone (free and total), folate, dehydroepiandrosterone sulfate (DHEAS), follicle-stimulating hormone, luteinizing hormone, progesterone, prolactin, estradiol, cancer antigen 125, and serum HCG. The UA&C and urine HCG were negative; all other results were within normal limits.
Operative Findings and Diagnosis
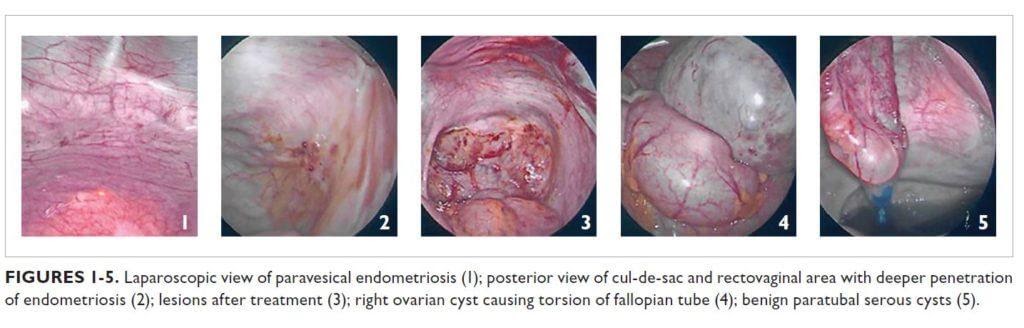
Operative findings were consistent with stage IV endometriosis. Laparoscopic visualization and histology revealed scattered superficial endometriosis on the anterior cul-desac and paravesical areas (Figure 1). The posterior cul-de-sac, uterosacral ligaments, and rectovaginal area had deeper lesions that penetrated through the fibroadipose tissue (Figure 2). The posterior aspect of the right ovary also contained vascular adhesions and endometriosis. All the lesions were treated with vaporization or excision techniques (Figure 3).
The large right ovarian cyst appeared benign but adhered to the pelvic sidewall and portions of the loops of bowel. The cyst caused partial torsion of the right fallopian tube (Figure 4) and compression over the loops of bowel, which led to partial distension of the bowels and sigmoid colon. The cyst was aspirated and found to contain a chocolate-like material. Ovarian cystectomy was performed and an endometrioma was confirmed by histologic evaluation of the specimen.
Further exploration revealed multiple benign paratubal serous cysts in the left fallopian tube (Figure 5); the largest of these was excised and the rest were aspirated. The left distal ureter also was involved, with dense adhesions that were lysed to restore normal anatomy. The left ovary contained multiple functional cysts.
The uterus was normal in shape and contour. Hysteroscopy with endometrial biopsy was performed, and no evidence of malignancy or hyperplasia was found. Peritoneal washing and cytology were free of malignancy.
Proctosigmoidoscopy and rectovaginal examination performed at the conclusion of surgery revealed resolution of the thickening and irregularity at the sites of endometriosis treatment. The patient recovered well, with resolution of symptoms.
Discussion
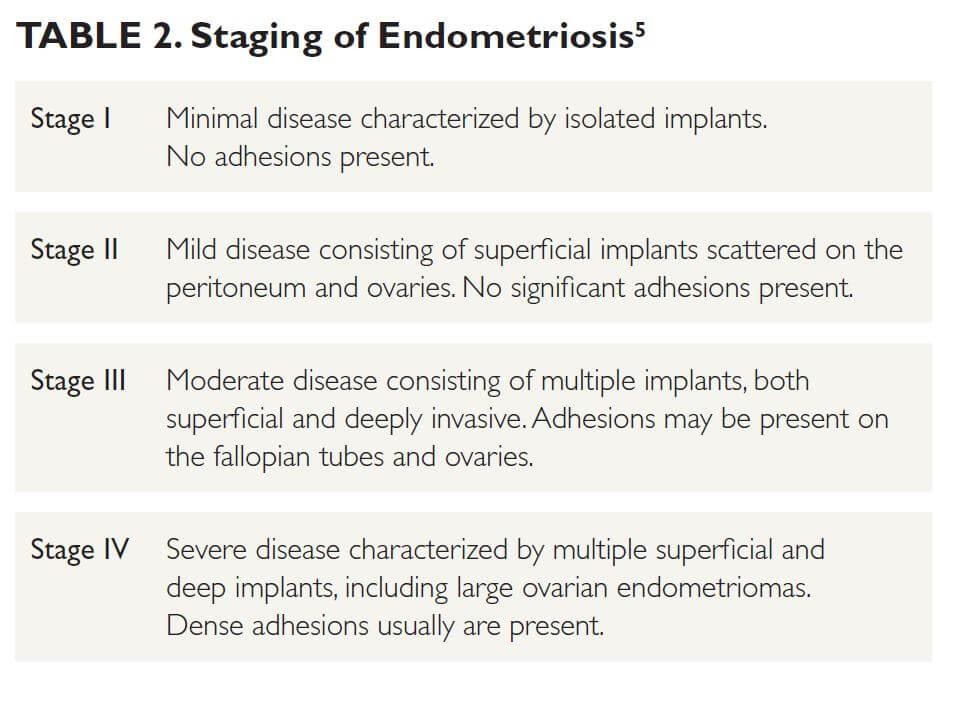
Endometriosis is a chronic inflammatory disorder caused by the growth of endometrial-like tissue outside the uterine cavity.1 The most common sites of endometriosis are the ovaries, uterosacral ligaments, posterior cul-de-sac, rectosigmoid colon, and bladder.2 It affects approximately 10% of women of reproductive age, with peak prevalence occurring in those aged 25 to 35 years.1,2 Risk factors include first-degree relatives with endometriosis, early menarche, short menstrual cycles, nulliparity, and low body mass index.3
The exact etiology of endometriosis is unclear, but several theories have been proposed. The most widely promoted is Sampson’s theory of retrograde menstruation. During this phenomenon, endometrial tissue enters the peritoneal cavity through the fallopian tubes and implants itself in the peritoneum and surrounding organs, leading to adhesions, inflammation, and chronic pain.1,4 However, retrograde menstruation is a normal occurrence in 90% of women and thus does not explain why only some develop endometriosis.2,3
More recent theories suggest immunologic dysfunction and genetic/epigenetic components are involved in the pathogenesis of endometriosis.3 Studies have shown that women with endometriosis appear to have lower cell-mediated immunity with decreased T-cell and natural killer cell responses, which may alter the ability of the immune system to target and destroy the endometriotic lesions.2,3 A third theory, the theory of Müllerianosis, suggests that during fetal organogenesis, Müllerian cells remain in the pelvis and differentiate into functioning endometrial glands and stroma under the influence of estrogen.2,3 Still, these theories fail to explain why the tissue of endometriosis has a similar histologic appearance to the endometrium yet functions differently.3
Although definitive diagnosis requires surgery, a presumptive diagnosis can be made from patient history, examination, and imaging. The hallmark finding on examination of a patient with endometriosis is tender, nodular thickening along the uterosacral ligaments and posterior cul-de-sac and a fixed, retroverted uterus. However, examination usually reveals nonspecific findings.2,3
Imaging is useful in both ruling out other causes of pelvic pain and in the preoperative assessment of the extent of disease. TVUS, the first-line imaging modality used in this setting, can detect endometriosis in the posterior cul-de-sac, bladder, and rectosigmoid area. Lesions appear as irregular thickening or hypoechoic nodules, and there may be free fluid in the cul-de-sac.3,8
TVUS also has the highest sensitivity and specificity in identifying ovarian endometriomas. These cysts commonly are referred to as “chocolate cysts” because they contain a thick, brown, bloody fluid. They tend to form dense adhesions on the peritoneal wall, bowels, and other surrounding structures. On TVUS, endometriomas classically appear as unilocular cysts, with homogeneous ground-glass echogenicity of the cystic fluid and poor vascular flow.3
There is no cure for endometriosis. The goal of treatment is to suppress pain. Medical therapies generally are offered first and include nonsteroidal anti-inflammatory drugs (NSAIDs) and combined oral contraceptive pills, progestins, danazol, or gonadotropin-releasing hormone analogs.3,4 These medications are intended to control pain by reducing inflammation, suppressing ovarian hormone production, and reducing menstruation.4
Operative laparoscopy is reserved for patients who do not respond to medical treatment or for those with endometriomas or infertility secondary to endometriosis. However, symptoms usually recur at a rate of 44% within 5 years after surgery.3 Therefore, it is recommended that patients begin hormone suppression postoperatively, if tolerable, to help prevent the recurrence of symptoms.4
Conclusion
In the primary care setting, the diagnosis of endometriosis is clinical, based on the patient’s history and physical examination findings. Although physical examination findings may be nonspecific, the presence of tenderness or nodular thickening along the uterosacral ligaments and posterior cul-de-sac or a fixed, retroverted uterus should prompt the diagnosis of endometriosis.
Medical therapy should be offered for symptomatic relief. According to the American Academy of Family Physicians, therapy should begin with NSAIDs, which can be followed by hormonal therapy if needed. If the patient fails medical therapy — or if the patient desires pregnancy — referral to a gynecologist for further evaluation is warranted.9
Patient education should also focus on therapy being suppressive, not curative.3,4 Discontinuation of medications and surgical intervention both carry the risk of symptom recurrence.1 If the providers treating the patient in this case had considered endometriosis as the root of her complaints, she may have received intervention much sooner.
Cristina Baldassarri, MPA, PA-C, is a physician assistant; E. Rachel Fink, MPA, PA-C, is a physician assistant at Augusta Urology Associates and an assistant professor in the Physician Assistant Program at Augusta University.
References
- Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261-275.
- Davila GW, Kapoor D, Alderman E, et al. Endometriosis. Medscape. https://emedicine.medscape.com/article/271899-overview. Updated July 25, 2018. Accessed October 31, 2020.
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol. 2018;131(3):557-571.
- Nezhat C, Vang N, Tanaka PP, Nezhat C. Optimal management of endometriosis and pain. Obstet Gynecol. 2019;134(4):834-839.
- American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817-821.
- Schenken RS. Endometriosis: pathogenesis, clinical features, and diagnosis. UpToDate. https://www.uptodate.com/contents/endometriosis-pathogenesis-clinical-features-and-diagnosishttps://www.uptodate.com/contents/endometriosispathogenesis-clinical-features-and-diagnosis.%20Updated June 1, 2020. Accessed October 31, 2020.
- Johnson NP, Hummelshoj L, Adamson GD, et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32(2):315-324.
- Hudelist G, Ballard K, English J, et al. Transvaginal sonography vs. clinical examination in the preoperative diagnosis of deep infiltrating endometriosis. Ultrasound Obstet Gynecol.2011;37(4): 480-487.
- Schrager S, Falleroni J, Edgoose J. Evaluation and treatment of endometriosis. Am Fam Physician.2013;87(2):107-113.




Add A Comment
You must be logged in to post a comment.